Question 11.4: A sample of gas has an initial volume of 158 mL at a pressur......
A sample of gas has an initial volume of 158 mL at a pressure of 735 mm Hg and a temperature of 34 °C. If the gas is compressed to a volume of 108 mL and heated to a temperature of 85 °C, what is its final pressure in millimeters of mercury?
Step-by-Step
The 'Blue Check Mark' means that this solution was answered by an expert.
Learn more on how do we answer questions.
Learn more on how do we answer questions.
GIVEN:
P_1 = 735 mm Hg
t_1 = 34 °C t_2 = 85 °C
V_1 = 158 mL V_2 = 108 mL
FIND: P_2
RELATIONSHIPS USED
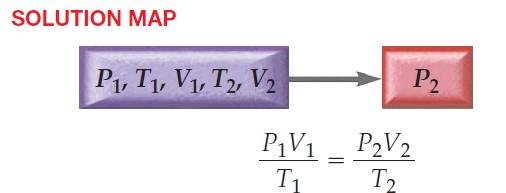
\frac{P_{1}V_{1}}{T_{1}}\;=\;\frac{P_{2}V_{2}}{T_{2}} (Combined gas law, presented in this section)
\frac{P_{1}V_{1}}{T_{1}}\;=\;\frac{P_{2}V_{2}}{T_{2}} \\ P_{2}=\frac{P_{1}V_{1}T_{2}}{T_{1}V_{2}} \\ \begin{array}{c}{{T_{1}=34\ +\ 273\ =\ 307~\mathrm{ K }}}\\ {{T_{2}=85\ +\ 273\ =\ 358~\mathrm{ K }}}\end{array} \\ P_{2}={\frac{735\;\mathrm{mm~Hg}\ \times\,158\;\mathrm{\cancel{mL}}\ \times\;358\;\mathrm{\cancel{K}}}{307\;\mathrm{\cancel{K}}\times108\;\mathrm{\cancel{mL}}}} \\ = 1.25 \times 10^3 mm HgThe answer has the correct units, mm Hg. The answer is reasonable because the decrease in volume and the increase in temperature should result in a pressure that is higher than the initial pressure.
Related Answered Questions
Question: 11.15
Verified Answer:
GIVEN:
P_1 = 855 mm Hg
V_1[/...
Question: 11.16
Verified Answer:
GIVEN:
n = 1.2 mol
V = 28.2 L
T = 334 K
FIND: P
RE...
Question: 11.14
Verified Answer:
GIVEN:
P_1 = 3.2 atm
V_1[/la...
Question: 11.13
Verified Answer:
GIVEN: 18.4 in. Hg
FIND: torr
RELATIONSHIPS USED
1...
Question: 11.12
Verified Answer:
GIVEN:1.24 L H_2
FIND: g H_2...
Question: 11.11
Verified Answer:
GIVEN:
294 g KClO_3
P = 755 mm Hg (...
Question: 11.8
Verified Answer:
GIVEN:
m = 0.311 g
V = 0.225 L
t = 55 °C
P = 886 m...
Question: 11.5
Verified Answer:
GIVEN:
V_1 = 4.8 L
n_1[/late...
Question: 11.7
Verified Answer:
GIVEN:
P = 24.2 psi
V = 3.2 L
t = 25 °C
FIND: n
RE...
Question: 11.6
Verified Answer:
GIVEN:
n = 0.845 mol
P = 1.37 atm
T = 315 K
FIND: ...